Table of Contents
Case Name: Hymowitz v. Eli Lilly & Co.
Court: Court of Appeals of New York
Year: 1989
Citation: 73 N.Y.2d 487, 539 N.E.2d 1069, 541 N.Y.S.2d 941
Introduction to Hymowitz v. Eli Lilly & Co.
Hymowitz v. Eli Lilly & Co. is a landmark 1989 New York Court of Appeals case that established the doctrine of market share liability for plaintiffs harmed by the drug diethylstilbestrol (DES). DES was a drug prescribed to pregnant women to prevent miscarriages but was later found to cause reproductive health issues in the daughters of women who took it. The Hymowitz case allowed plaintiffs to recover damages from DES manufacturers without having to prove which company produced the specific pills responsible for their injuries. Instead, manufacturers were assigned liability based on their share of the national DES market.
The Hymowitz ruling significantly shaped product liability law regarding generic drugs and consumer safety. By easing the burden of proof for plaintiffs, it facilitated recovery for DES victims and influenced pharmaceutical regulations. The market share theory from Hymowitz has been adopted in other jurisdictions and applied to cases beyond DES. However, it remains controversial. Overall, Hymowitz v. Eli Lilly & Co. was a groundbreaking decision with far-reaching legal and policy implications.
Background on DES
DES, or diethylstilbestrol, is a synthetic form of estrogen that was prescribed to pregnant women between the 1940s and 1970s to prevent miscarriages and other pregnancy complications. An estimated 5-10 million pregnant women and their children were exposed to DES before it was pulled from the market.
DES was later found to cause serious medical issues in those exposed to it. Daughters exposed to DES in utero were at increased risk for vaginal cancer, infertility, pregnancy complications, and breast cancer. The sons exposed had higher rates of genital abnormalities, testicular cancer, and infertility. DES was a tragedy that exposed millions to harmful effects before being recognized as dangerous.
Background and History of the Case
The Hymowitz v. Eli Lilly & Co. case began when Enid Hymowitz filed a lawsuit against Eli Lilly and other manufacturers of diethylstilbestrol (DES) in New York state court in 1982. She alleged the companies were negligent in designing, testing, inspection, labelling, marketing, and promoting DES. DES was a synthetic form of estrogen prescribed to pregnant women between 1938 and 1971 to prevent miscarriages. Still, it was later found to cause rare vaginal cancer and other severe issues in female offspring exposed in utero.
Hymowitz was one of many plaintiffs who had difficulty identifying the specific manufacturer of the DES taken by their mothers due to the generic nature of the drugs and the number of producers. The trial court dismissed the claims based on the statute of limitations. However, the intermediate appellate court reinstated the claims based on New York’s revival statute for DES suits. The New York Court of Appeals then took up the case to address the issue of identifying liable defendants.
Legal Issues Addressed
The key legal issue in Hymowitz v. Eli Lilly & Co. was the difficulty plaintiffs faced in identifying the specific manufacturer responsible for their injuries caused by DES. DES was a drug produced from a generic formula, and manufacturers did not distinguish their products through trademarks or product appearance. As a result, plaintiffs could not prove which company produced the DES taken by their mothers.
To address this, the New York Court of Appeals adopted the theory of market share liability. Under this doctrine, defendants would be liable based on their share of the DES market rather than requiring plaintiffs to identify the specific manufacturer. The rationale was that liability should be apportioned according to the defendants’ participation in creating the risk. This allowed plaintiffs to recover damages despite being unable to pinpoint the exact producer responsible for their injuries.
Ruling and Rationale
The New York Court of Appeals ruled in Hymowitz v. Eli Lilly & Co. on July 6, 1989. In a 4-2 decision, the court affirmed the appellate court’s denial of summary judgment for the defendant manufacturers. The ruling adopted a national market share liability theory to determine the defendants’ liability and apportion damages.
The court acknowledged the challenge faced by plaintiffs in identifying the specific manufacturer responsible for their injuries decades after exposure. Traditional tort doctrines of concerted action and alternative liability were found inadequate to address this issue. The court thus turned to the market share theory as a fair solution that apportioned liability based on the defendants’ national market share of DES sold for pregnancy use. This allowed plaintiffs to recover from defendants in proportion to their market share without needing to identify the exact manufacturer.
The court justified this novel approach by emphasizing the public policy goal of holding accountable those who profited from a defective product. The ruling noted that between an innocent plaintiff and negligent defendants, the manufacturers were in a better position to absorb the cost of injury. The market share theory balanced the interests of DES plaintiffs and defendants.
Impact on Future Cases
The Hymowitz v. Eli Lilly & Co. decision significantly influenced how future DES cases were handled. By establishing market share liability, it provided a pathway for plaintiffs who could not identify the specific manufacturer responsible for their injuries to still potentially recover damages.
This enabled numerous plaintiffs in subsequent DES lawsuits to bring claims that may not have been possible previously. Hymowitz set a precedent that was followed by other courts presiding over DES cases in New York and elsewhere.
More broadly, Hymowitz shaped the evolution of product liability law across the United States. It demonstrated that traditional tort law doctrines like causation could be adapted to address challenging scenarios where plaintiffs lacked proof of direct causation. The market share theory was an innovative approach to liability that aligned with the prevailing notion that manufacturers should be responsible for product-related harms.
While controversial, Hymowitz opened the door for considering alternative liability frameworks beyond traditional negligence and strict liability rules. The market share approach has been extended and modified over time to suit different situations. Hymowitz was a landmark case that demonstrated the flexibility and capacity of tort law to develop new remedies in response to modern products and injuries.
Criticisms
As established in Hymowitz v. Eli Lilly & Co., market share liability has faced criticism on several fronts. One of the main problems cited is that it holds manufacturers liable based on their market share rather than requiring plaintiffs to prove causation. This goes against traditional tort principles that defendants should only be liable if they caused the plaintiff’s injury. Holding companies liable based on their overall market share has been called an “arbitrary” and “unprincipled” approach by critics.
Related to this, some argue that market share liability unfairly burdens companies with larger market shares simply due to their success in the market. Smaller companies with minimal DES sales may completely avoid liability, while larger companies bear the costs. This raises concerns about fairness and due process. Companies are forced to pay damages without proof that their products have injured the plaintiff.
Another concern is that market share liability reduces incentives for product safety. If manufacturers know they will be liable based on market share regardless of defectiveness, critics argue they have less incentive to ensure their products are safe. This could negatively impact consumer safety over time.
Overall, the market share approach remains controversial. While it aided DES plaintiffs by easing the burden of proof, critics argue it strained traditional causation principles in tort law and may have unintended consequences. The debate continues over whether market share liability achieves justice or overreaches doctrinal boundaries.
Comparison to Sindell v. Abbott Laboratories
The Hymowitz v. Eli Lilly & Co. case has often been compared to the landmark Sindell v. Abbott Laboratories case from 1980, which also involved DES plaintiffs unable to identify the specific manufacturer responsible for their injuries. There are several key similarities and differences between the two cases:
- Both Sindell and Hymowitz grappled with the issue of how to hold manufacturers liable when plaintiffs could not prove traditional causation due to the generic marketing of DES.
- Sindell established the “market share liability” doctrine, allowing plaintiffs to join defendants representing a “substantial share” of the DES market. Hymowitz built upon this by modifying the approach to use national market share percentages.
- While Sindell apportioned liability based on each defendant’s share of the California market specifically, Hymowitz used national market share, recognizing the nationwide marketing of DES.
- The Hymowitz court rejected exculpation for defendants who could prove they did not manufacture the DES taken by a particular plaintiff, finding it unfair to place the burden on plaintiffs. Sindell had allowed this defence.
- Both cases aimed to balance fairness and justice for plaintiffs against defendants’ rights. However, Hymowitz placed more emphasis on facilitating plaintiff recovery.
- Hymowitz explicitly rejected the idea that market share liability requires proof of concerted action or joint control of risk among defendants, which was hinted at in Sindell.
So, while Sindell v. Abbott Laboratories paved the way by establishing market share liability, Hymowitz v. Eli Lilly & Co. built upon and modified the doctrine to craft an approach more favorable for DES plaintiffs. The Hymowitz framework would influence subsequent DES cases and shape market share liability principles.
Implications for Pharmaceutical Companies
The ruling in Hymowitz v. Eli Lilly & Co. had significant implications for pharmaceutical companies and how they approach product liability for drugs. By establishing the market share liability theory, the case increased potential liability for all manufacturers of generic pharmaceuticals like DES. Companies could now be held financially responsible for injuries caused by their products, even if the specific manufacturer was unknown or impossible to identify.
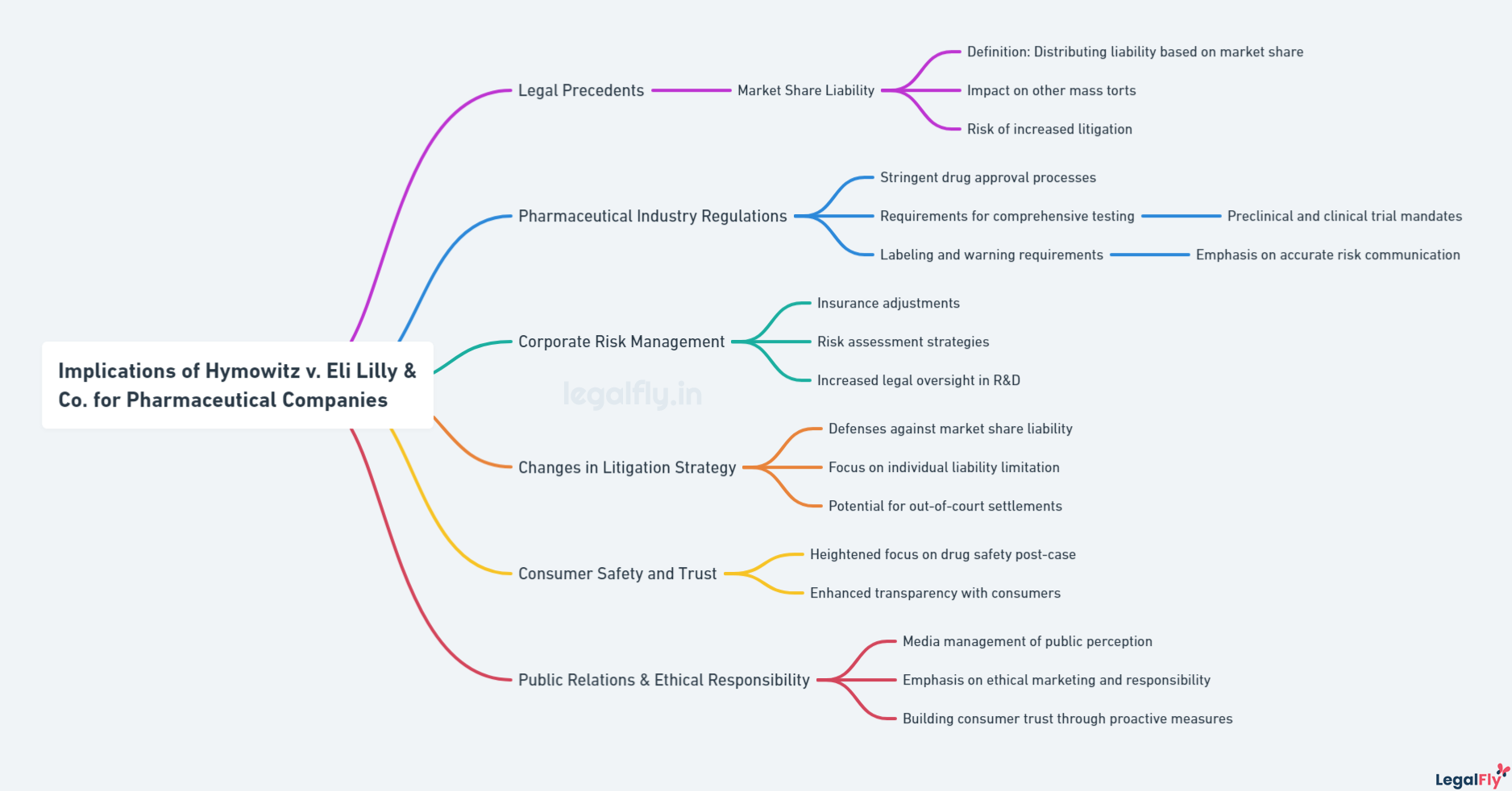
The prospect of greater liability pressured drug makers to take a more cautious approach when bringing new products to market. Companies now had to consider long-term risks, even decades into the future, when their products might produce injuries that could lead to litigation. This necessitated more rigorous safety testing and evaluation before releasing new drugs.
The Hymowitz decision also impacted how companies marketed and promoted pharmaceuticals. More restraint was required to avoid overstating efficacy claims or understating potential side effects. Truthful and ethical marketing practices have become even more essential in avoiding liability for misrepresenting drug risks and benefits to consumers and physicians.
Overall, the market share approach substantially raised the stakes for pharmaceutical companies by linking liability to market share rather than specific causation. Hymowitz made clear manufacturers could not escape responsibility for the harm caused by their products. This compelled more informed business and research practices within the industry.
Conclusion
The Hymowitz v. Eli Lilly & Co. case established the novel doctrine of market share liability, allowing plaintiffs to recover damages from DES manufacturers without having to identify the specific company responsible for their injuries. This landmark ruling fundamentally changed product liability law by prioritizing victim compensation over causation principles.
Key takeaways from the case include:
- Market share liability relaxes the identification requirement for plaintiffs, shifting the burden to defendants to exculpate themselves. This makes it easier for victims to recover damages.
- Companies can be held liable based on their share of the relevant product market, not on definitive proof they caused harm. This promotes accountability and fairness.
- Innovative doctrines like market share liability are sometimes necessary when traditional tort rules fail to provide justice. The court recognized the challenges faced by DES plaintiffs.
- Pharmaceutical companies must be vigilant in testing and warning about potential side effects, even decades after a drug goes to market. The impacts may only become evident over time.
Though the Hymowitz case dealt specifically with DES, its implications are far-reaching. The judgment demonstrated a willingness to adapt tort law to address novel, complex public health issues. It set an influential precedent for using market share concepts in mass tort litigation. Even decades later, the Hymowitz decision remains a landmark ruling and a striking example of judicial creativity in the service of equity. Its spirit continues to shape ongoing debates about victim compensation and corporate responsibility.